Unit 4 - We've got the power
Content
- 9u4.1 - Energy stores
- 9u4.2 - The law of conservation of energy
- 9u4.3 - Sankey diagrams
- 9u4.4 - Efficiency
- 9u4.5 - Energy transfer: conduction, convection and radiation
- 9u4.6 - Conductors and insulators
- 9u4.7 - Greenhouse effect
- 9u4.8 - Homeostasis of temperature regulation in humans
- 9u4.9 - Energy generation
- 9u4.10 - Now test yourself
Scheme of work
9u4.1 - Energy stores
Energy transfer can do useful work. Although this sounds odd, it just means that by transferring energy from one kind to another we can benefit from the change in some way.
For example, the transfer of energy from electrical to light can be used to illuminate our homes. The transfer of nuclear energy to electrical energy can be used to power a submarine.
Energy can be subdivided into two categories
- Kinetic
- Potential
Kinetic energy
Kinetic energy is the energy that something has stored as motion. Things of all sizes possess kinetic energy when they move. The motion of particles on a microscopic level is also called heat energy.
Potential energy
This covers many energy stores that are due to the nature or position of something, which can be transformed into another form of energy. Examples are shown in the list below:
- Gravitational potential energy
- Chemical energy
- Elastic energy
- Nuclear energy
- Electrical energy
- Electromagnetic wave energy
Activity - Energy comic book
Students make a comic where they include as many energy types as possible.
9u4.2 - The law of conservation of energy
This is probably the most important concept regarding energy. It can be neither created nor-destroyed. This means that energy can simply be tranformed from one type to another. One utility of this law is that we can measure energy change for certain types of energy and relate them to other types of energy.
Kinetic energy
The amount of kinetic energy that an object has depends on both its velocity and mass. The actual relationship is:
Kinetic energy = ½ x mass x (velocity)2
Example: Determine the kinetic energy of a football with mass 2kg travelling at 20ms-1
Kinetic energy = ½mv2
Kinetic energy = 0.5 x 2 x 20 = 20 Joules
Gravitational potential energy
The amount of GPE that an object has depends on its height above the ground, the gravitational constant and its mass.
The gravitational constant, g, depends on the planet, but here on earth it is 9.81 ms-2.
The actual relationship is:
Gravitational potential energy = mass x gravitational constant x height
Example: Determine the gravitational potential energy of a parachutist with mass 80kg at a height of 1000m
Gravitational potential energy = mass x g x height
Gravitational potential energy = 80 x 9.81 x 1000 = 784.8 kiloJoules
Notice that in the above examples the mass is always measured in kilograms and the distance in metres. These are the SI units that should always be used.
Investigation - Trolley and ramp experiment
Use the trolley and ramp to investigate the effect ramp height on the kinetic energy of the trolley.
Calculate velocity at bottom of ramp for a few different ramp angles/heights using the last 5 dots from the ticker-timer tape. Use the velocity and the mass of the trolley to calculate the kinetic energy. The gravitational potential energy can be determined from the height of the trolley and its mass.
9u4.3 - Sankey diagrams
The data provided in the previous example shows us that not all of the gravitational potential energy is tranferred to kinetic energy. This is a common factor with most energy transfers - not all of the input energy is output as useful energy. Some is always lost, usually as heat.
This transfer of energy to both useful and lost energy can be represented using a Sankey diagram.
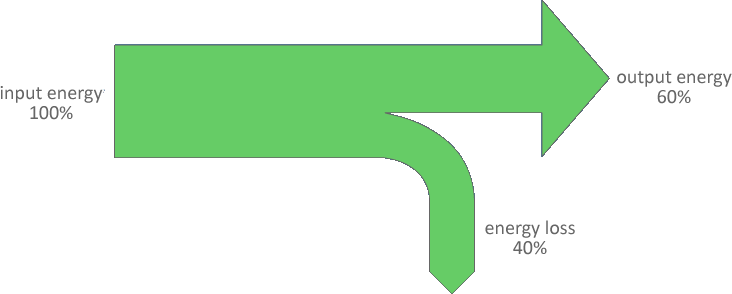
The Sankey diagram is drawn to scale, with the width of each section proportional to the amount of energy at that point. Hence in the diagram above, the width of the arrow labelled output energy measures exactly 6/10 of the width labelled input energy.
9u4.4 - Efficiency
Determining efficiency
The Sankey diagrams in the previous section are a visual representation of the amount of useful energy is obtained during an energy transfer. This can also be expressed as a numerical value called the efficiency of the transfer. If all of the input energy is useful then the transfer is 100% efficient (this is virtually impossible).
There is a simple equation that allows us to calculate the efficiency of an energy transfer:
Efficiency = useful energy/input energy
This could also be expressed as a percentage efficiency
Percentage efficiency = 100 x useful energy/input energy
9u4.5 - Heat energy transfer
The nature of heat
The term "heat" refers to the particle vibrations in matter. The particles themselved have kinetic energy as they are moving, but in the macroscopic world we experience this as heat.
Measurement of heat
Heat is measured using a thermometer. The absolute temperature is directly proportional to the average kinetic energy of the particles. Take a look at this model of Brownian motion. You will see that the particles are not all moving at the same speed. This is because of the random nature of particular motion. However, the average speed can be used to determine the absolute temperature (this goes beyond our scope here).
We just need to know that the average kinetic energy of the particles is directly proportional to the temperature in Kelvin. Remember that 0 Kelvin = -273 °Celsius. We can get the temperature in Kelvin by adding 273 to the temperature in Celsius.
Absolute temperature = 273 + °Celsius
Measuring heat energy change in materials
All substances absorb heat energy in different ways, meaning that the temperature change in a material is not only dependent on the energy absorbed and the mass, but also on the nature of the absorbing medium.
Water has a very high capacity for absorbing heat energy. 1kg of water must absorb 4.2 kJ of energy to increase the temperature by 1 °C.
We say that water has a specific heat capacity of 4.2 kJ kg-1 K-1
Determine the temperature change when 10 kg of water absorb 500 kJ of energy
The energy absorbed = mass (in kg) x specific heat capacity of water x the temperature change.
ΔE = mcΔT
Rearranging:
ΔT = ΔE/mc
ΔT = 500 /(10 x 4.2) = 11.9 °C
Specific heat capacity
The specific heat capacity of any substance is the amount of heat energy in kJ that is needed to change the temperature of that substance by 1 °C.
Water has a specific heat capacity of 4.2 kJ kg-1 K-1 (This value will always be given to you in any test situation).
Investigation - Heat energy transfer from a spirit burner
Spirit burners can be used to transfer heat into a beaker of water. If we know the theoretical energy released by the spirit burner, then we can determine the efficiency of energy transfer.
Conduction, convection and radiation
Heat gets transferred by three processes, depending on the state of matter
- Conduction (solids)
- Convection (liquids and gases
- Radiation (all states)
Conduction
The particles in vibrating solids pass their vibrations along to particles that are vibrating less.

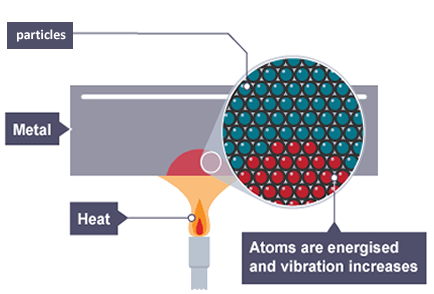

Investigation - The heat conductivity of several metals
For this experiment you will use heat sensitive nail varnish that changes colour when the temperature of the metal reaches about 30°C.
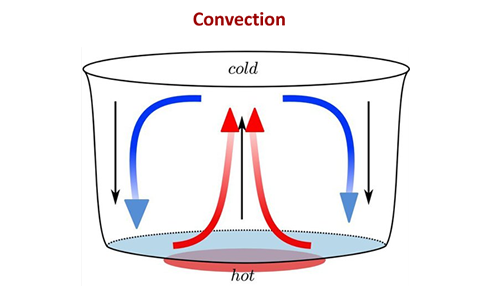
Convection
Particles in fluids are able to move around with respect to one another and, as such, do not pass vibrations very easily. They are poor conductors of heat. However, the vibration of particles in fluids causes them to spread farther apart. This means that the hot fluid is less dense (less mass per unit volume), and tends to rise as more dense material falls to take its place. This is called convection
In fluids (liquids and gases) convection currents are set up that carry hot regions of the fluid upwards while cold (denser) regions of the fluid fall downwards.
Convection of hot air from the equatorial regions of the Earth when combined with the rotation of the Earth produces the weather patterns. The hot air rises and is replaced by cooler air from nearer the poles. This produces a region of very high winds, known as the roaring forties, after the latitude 40°.
Land tends to heat up more easily than water, and so convection currents often form at coastlines on hot days.
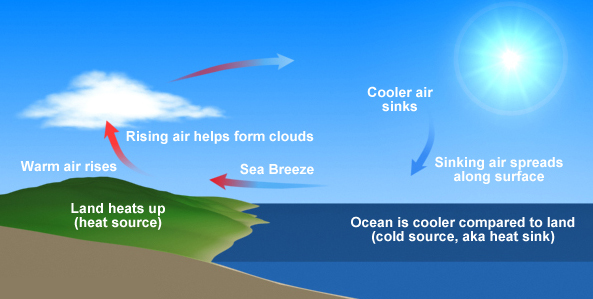
On a smaller scale, convection also drives hot-air balloons. The heat source fills the balloon with hot air by convection, which is less dense than the air that surrounds the balloon. The balloon is pushed upwards by the coolder, more dense air.

Radiation
All hot bodies emit electromagnetic radiation in infra-red region of the electromagnetic spectrum. We usually call this radiation, heat rays, which is slightly confusing in that we have already defined heat as the vibration of the particles of matter.
However, when heat radiation interacts with matter it causes the particles to vibrate more, heating them up. This is where the confusion arises - one causes the other.
We can feel this heat transfer simply by standing in the sunshine. The sun is 150 million km from the earth, but we still feel the heat arriving. It travels through empty space in the form of electromagnetic radiation. In fact, it takes about 8.3 minutes to arrive at the Earth.
Revision opportunity
In the previous unit we learned about electromagnetic radiation, which travels at 3.0 x 108 ms-1 through a vacuum (empty space).
The sun is 150 million km away, this equals 150 x 106 km = 150 x 150 x 109m = 1.5 x 1011m.
velocity = distance/time
The time taken to travel a distance = distance/velocity = 1.5 x 1011m/3.0 x 108 = 500s
9u4.6 - Conductors and insulators
As we have already learned, different materials conduct heat by different amounts. We call substances that do not allow the transfer of heat by conduction "insulators" and substances that do allow the transfer of heat by conduction "conductors".
Insulators are very important in situations where heat losses are not required. Think about your clothes on a winter's day. You would not want them to conduct heat away from your body. You would want them to be good insulators.
We can find out the insulation properties of materials by investigation.
Experiment to determine the insulation properties of some materials
A beaker of hot water can be surrounded by different materials and the heat losses in the beaker measured using a thermometer.
9u4.7 - Greenhouse effect
The Earth's atmosphere
The air is made up of mainly nitrogen gas (79%), oxygen gas (20%) and argon gas (1%). However, there are other gases present in smaller amount, such as carbon dioxide (0.02%), water vapour and methane (0.00017%). These trace gases play an important role in temperature control on the Earth.
Carbon dioxide and methane gases allow the sunlight through to the Earth's surface, but when the Earth heats up it radiates heat back out towards space. The carbon dioxide and methane absorb this heat radiation and prevent it leaving. This keeps the Earth warm. It is called the Greenhouse Effect and the gases that cause this are called Greenhouse Gases.
Greenhouse Gases
- Water vapour, H2O
- Carbon dioxide, CO2
- Methane, CH4
Water vapour is the major contributor to the greenhouse effect.
It is a Greenhouse Gas, but human activity does not contribute to its influence on the climate. When we discuss greenhouse gases, we normally focus on those gases that are produced by human activity (anthropogenic).
Water vapour in the atmosphere is only affected by the temperature of the environment. As the global temperature rises, the amount of water vapour in the atmosphere increases, increasing its greenhouse effect. This is called a positive feedback mechanism.
It is thought that something of this kind occurred in the past on the planet Venus, which resulted in the extremely dense atmosphere and high temperatures.
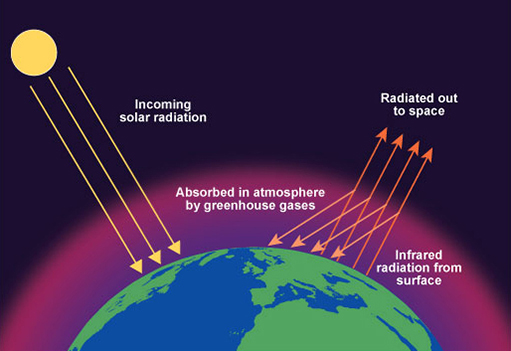
Global warming
With the arrival of the industrial age, the amount of fossil fuels burned for useful energy transfer (for example, to drive machinery) increased a great deal. This has had the effect of increasing the amount of carbon dioxide in the atmosphere which has lead to a steady increase in the average temperature of the Earth with corresponding climate change.
The consequences of increased temperature are as follows:
- Melting polar ice
- Melting glaciers in mountains
- Heating up the oceans
- Destroying habitats
- Desertification
- Extreme weather events
If the Earth's temperature increases by only a few degrees there will be devastating consequences for billions of people and other organisms.
Solutions to global warming
There is a general consensus among the scientific community that the amount of carbon dioxide in the atmosphere must be either stabilized or reduced. There are several ways that this can be done, but it requires the cooperation of governments all over the world.
- Reduce fossil fuel emissions
- Sequestration (capture) of carbon dioxide in the atmosphere
- Increasing global tree coverage
The first item on the list is the most controvertial, as the most industrialised nations are the worst offenders and there is not the political will to reduce their energy expenditure. Likewise, some of the developing countries do not want to hinder their economy by more expensive energy generation. There needs to be an agreement whereby the richest nations subsidise the poorer nations in terms of renewable energy generation to encourage them to not use fossil fuels. Unfortunately, politicians and scientists have different agendas.
9u4.8 - Homeostasis of temperature regulation in humans
Homeostasis literally means "keeping everything the same". It refers to the factors that balance an organisms requirements for nutrition and temperature.
In general, organisms function most efficiently at about 37°C. This is because it appears to be the ideal temperature for enzyme driven reactions to occur at the correct rate. Human beings are no exception.
However, due to the zero'th law of thermodynamics we are in a constant battle to maintain body temperature. If our surroundings are too hot we need to cool down. If our surroundings are too cold we need to heat up. All of this is done by the autonomous nervous system.
Keeping warm
The body recognises the signs of losing too much heat to the surrondings and sets in motion a series of activities designed to produce more heat and limit heat loss.
- Shivering
- Blood vessels near skin constrict
- Hair muscles erect
Shivering uses muscles that generate heat as a by-product of activity.
Constriction of blood vessels near the surface of the skin reduces heat losses by cooling the skin.
Arrector pili muscles stand the hairs on the skin up so that they can trap more air between them. This prevents heat losses as the trapped air is a poor conductor of heat.
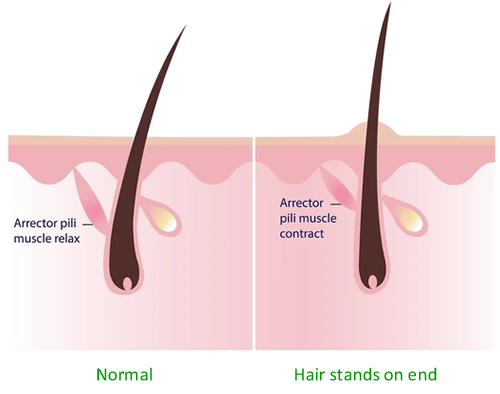
Keeping cool
The body recognises the signs of gaining too much heat from the surroundings and sets in motion a series of activities designed to lose heat.
- Sweating
- Blood vessels near skin expand
- Hair muscles relax
Sweating puts a layer of water on the skin. The most energetic water molecules can then evaporate reducing the average energy of the particles at the skin. The skin cools down.
Expanding the blood vessels at the surface brings warm blood near the surface allowing more efficient radiation for heat energy loss.
Experiment - The penguin huddle
This experiment examines the relationship between size and temperature control.
9u4.9 Energy generation
As we have seem earlier, energy cannot be "generated" as it can be neither created nor destroyed. However, the term "energy generation" refers to the transfer of energy from one form to electrical energy that we use in a modern society for heat, light, transport and industry.
The electrical energy is transported to industry and homes via the electrical grid. This is an interconnected series of cables that carry the electricity from the source to its destination.
Electricity generation stations
These are where the original energy source is transformed into electrical energy. There are several renewable and non-renewable sources of energy.
- Fossil fuels (coal, oil and natural gas)
- Solar energy
- Wind energy
- Tidal energy
- Hydroelectric
- Geothermal
- Nuclear fission
The electrical power grid
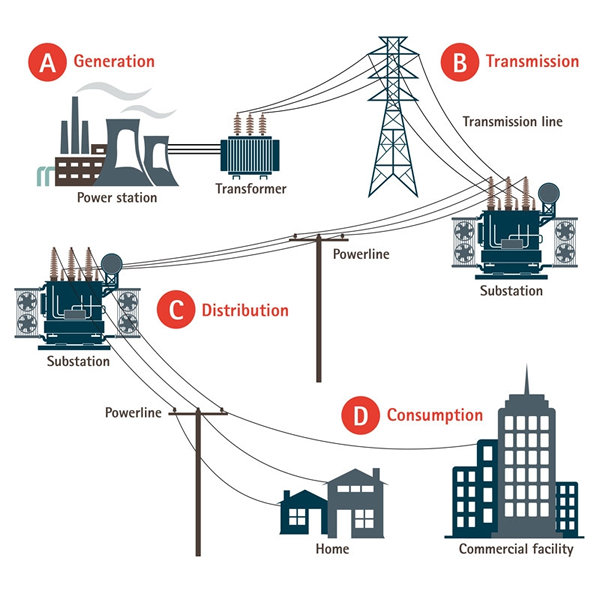
The electricity is generated in the power stations at about 25,000 volts (push), which is not suitable for transmission over large distances. It goes first to a sub-station that steps the voltage (push) up to typically 400,000 volts. It then passes through thick cables made of copper and aluminium with steel bindings (copper and aluminium are very good electrical conductors, while steel provides the strength), and relayed via pylons to sub-stations that step the voltage down to levels more suitable for industry and the home, between 220 and 240 volts.
Sources of energy
The students are to investigate the sources of energy outlined above and prepare a PowerPoint presentation of ONE of the energy sources, highlighting its advantages and disadvantages.
9u4.10 - Now test yourself
Click on the button below to access the self-tests for MYP9 and MYP10.