Unit 1 - Water, water everywhere
Content
- 9u1.1 - The scientific method
- 9u1.2 - Kinetic theory
- 9u1.3 - Solids, liquids & gases
- 9u1.4 - Changes of state
- 9u1.5 - Pure substances and mixtures
- 9u1.6 - Solutions
- 9u1.7 - Separation techniques
- 9u1.8 - Elements and compounds
- 9u1.9 - The periodic table
- 9u1.10 - Metals and non-metals
- 9u1.11 - Now test yourself
Scheme of work
9u1.1 - Introduction to science - the scientific method
Science is the process that we use to answer questions about the world around us. There is nothing secret or magical or difficult about it.
The procedure
The first step is always making an observation. After seeing or observing something, we might ask a question, such as what, why, how many, how, when etc.
It is possible at this point that the observer may try to suggest an answer based on previous knowledge, or scientific deduction - we call this the hypothesis.
Then experimentation takes place to seek information (data) that helps to either support the hypothesis or leads to a different conclusion.
Summary of the scientific method
- Observation
- Question
- Hypothesis
- Fair test
- Record data
- Conclusion
- Evaluation
Things to note
1. A hypothesis has little value unless it is based on previous knowledge
2. A fair test (the actual investigation) means that all aspects (variables) that could affect the data have been correctly controlled
3. Data may be qualitative or quantitative
4. Quantitative data must be recorded with units of measurement
9u1.2 - The kinetic particle theory
The kinetic theory is a fundamental scientific theory that explains the behavior of matter. This theory provides a particle-level explanation of the macroscopic properties of the matter.
Important points of Kinetic Theory
- Gas Particles are Small and Far Apart: The actual volume of the particles in a gas is negligibly small compared to the volume the gas occupies. Most of the volume of a gas is empty space.
- Random Motion and Collisions: Gas particles are in constant random motion and move in straight lines until they collide with each other or with the walls of their container. These collisions are considered to be perfectly elastic, meaning there is no net loss of kinetic energy in the collisions.
- No Attractive or Repulsive Forces: Gas particles exert no forces on each other except during collisions. This means there is no attraction or repulsion between particles except at the instant of collision.
- Kinetic Energy and Temperature: The average kinetic energy of particles is directly proportional to the absolute temperature of the particles. This means that increasing the temperature will increase the particles' kinetic energy.
- Liquids: The particles are close together but have enough energy to allow them to move around in the body of liquid. Some particles gain enough energy to escape from the liquid as a gas.
- Solids: The particles are close together, but their energy is not enough to allow them to move around. The only motion that they are allowed is vibration, as the forces holding them in place are too strong.
The microscopic particles in solids, liquids and gases.
The macroscopic volume and shape properties in solids, liquids and gases.
Experiment: How many drops of water fit onto a coin?
In this experiment you will see how many water drops you can place on a coin without them spilling off the side.
9u1.3 - Solids, liquids & gases
Evidence for the kinetic theory - circus
- Brownian motion
- Marbles in a tray
- Potassium manganate(VII) crystal in water
- Diffusion (ammonia litmus)
- Compression of gases, liquids and solids
9u1.4 - Changes of state
States of matter /Changes of state
- Vaporisation
- Condensation
- Solidification
- Melting (fusing)
- Sublimation
Demonstration: Heating curve of ice
This requires ice from the freezer at sub-zero temperatures. A heat probe can be connected to logger-pro and the screen projected as the ice melts.
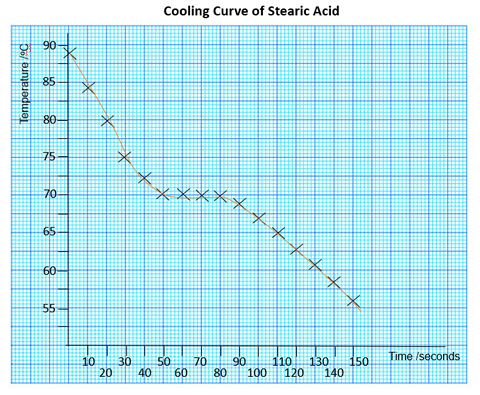
Experiment: Cooling curve of molten substance
The students will be provided with some molten (melted) pure substance, either stearic acid, lauric acid, vanillin or naphthalene in a clean test tube in a hot water bath at about 90º.
The students are to monitor the temperature using a thermometer while the tube stands in a beaker with room temperature water.
The temperature is recorded every 10 seconds, while the molten liquid is stirred using the thermometer.
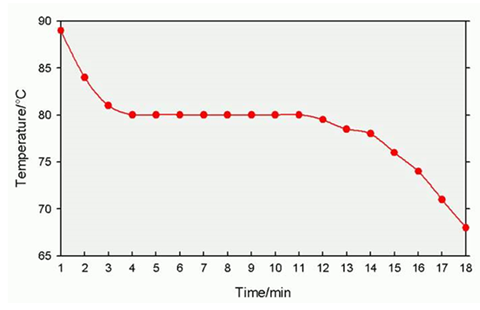
The cooling curve of a hot molten liquid explained:
The cooling curve above has three distinct sections:
- Section 1. From t = 0 to t = 4m
- Section 2. From t = 4 to t = 11m
- Section 3. From t = 11 to t = 18m
Section 1
The molten liquid is hotter than the surroundings and transfers heat energy to the surroundings. This is why is cools down from 96ºC to 80ºC.
Section 2
The substance is still transferring heat energy to the surroundings, but now it is also generating heat energy from chemical energy, as the hot liquid starts to solidify. The particles come closer together and transform chemical energy into heat energy. This is why the temperature remains constant.
Section 3
The substance has completely solidified and is now cooling once again by transferring heat to the surroundings.
9u1.5 - Pure substances and mixtures
Pure Substances
A pure substance is a material with a uniform and definite composition. It can be either an element, which consists of a single type of atom, or a compound, which consists of two or more types of atoms chemically bonded together. Pure substances have consistent properties throughout the sample and cannot be separated into other substances by physical means. Examples include distilled water, gold, and carbon dioxide.

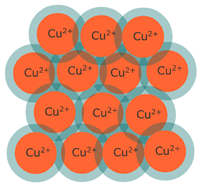
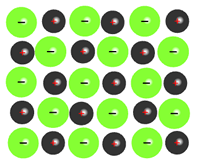

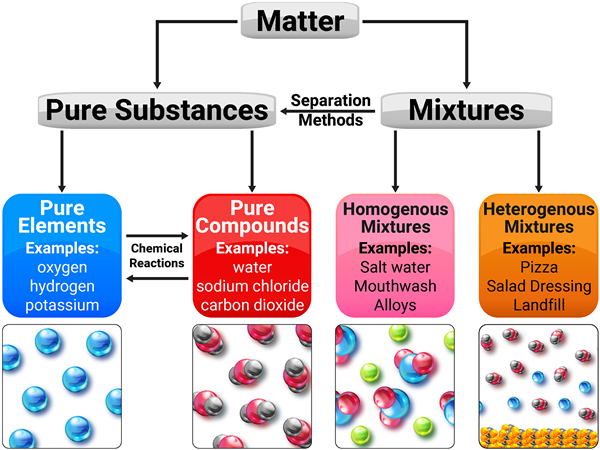
Mixtures
Mixtures consist of two or more substances physically combined. Unlike pure substances, the components of a mixture can vary in their proportion and can be separated by physical means such as filtration, distillation, or magnetic attraction.
Mixtures are categorized into homogeneous and heterogeneous mixtures:
- Homogeneous Mixtures: Also known as solutions, these mixtures have the same uniform appearance and composition throughout. Common examples include salt water, air, and vinegar.
- Heterogeneous Mixtures: These mixtures consist of visibly different substances or phases. The different components are physically distinct and can often be seen as individual substances. Examples include sand and iron filings, salad, or oil and water.
Key Differences
Understanding the distinction between pure substances and mixtures is fundamental in chemistry and helps in determining the appropriate methods for separating components or identifying materials based on their properties. While pure substances have fixed boiling and melting points, mixtures exhibit a range depending on the ratio of components and their individual properties.
Summary of pure substances and mixtures
Can you define the following words?
- Element
- Compound
- Mixture
- Homogeneous
- Heterogeneous
9u1.6 - Solutions - very special mixtures
A solution is a very special homogeneous mixture where the dissolved phase (the solute) is so finely dispersed that it is not visible.
Solutions are defined according to the mass of solute dissolved in a known volume of the solution, or the mol of solute in a known volume of solution.
Water properties
- Surface tension
- Solvent
- Polarity
- Density
- Specific heat capacity.
Experiment: To investigate factors that affect solubility
How much solute dissolves in a solvent at different temperatures?
9u1.7 - Separation techniques
Separation of mixtures can be performed in the laboratory using a variety of techniques, depending on the type of mixture involved.
- Decanting
- Filtration
- Distillation
- Chromatography
Here we are going to see how these techniques can be applied to several different types of mixture and how they work.
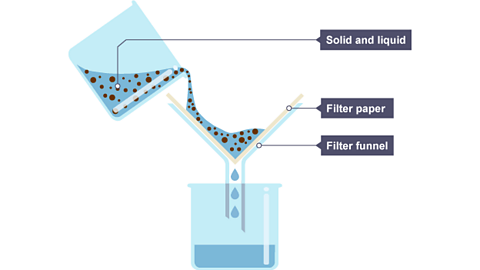
Solid mixed in a liquid
This can be separated by either decanting or filtering, depending on the type of solid. If it is very coarse grained then simply pouring off the liquid (decanting) can be used. However, if the solid is undissolved but mixed with the liquid then filtration can be used.
Filtration
Solid dissolved in a liquid
These components can be separated by distillation. Liquids can be heated until the boiling point and the gas produced lead to a cooling tube where it turns to liquid again. This allows the liquid component of the mixture to be collected.
A good example is the separation of a salt water mixture into pure water and salt
Distillation

If the liquid component is not required, then evaporation may be used. In this procedure the solution is allowed to stand for a period of time (often days) until the solvent (the liquid component of the solution) evaporates away.
Experiment: Rate of evaporation
In this experiment you will investigate the factors that affect the rate at which evaporation occurs in a solution.
9u1.8 - Elements and compounds
Elements
Elements are the fundamental building materials of matter. They consist of many billions of identical particles. These particles may be single atoms (from the Greek: atomos = indivisible) or found in pairs, in groups or all joined together.
Where there is more than one atom in a particle, these are called molecules. The gaseous elements usually consist of atoms in pairs, but there are exceptions, such as the noble gases (group 8)
Each element is represented by a symbol. This symbol either consists of one upper-case letter, or one upper-case letter followed by a lower-case letter.
Compounds
Substances made up of more than one element. The smallest particles may be molecules, each containing more than one type of atoms, or large structures made up of two or more types of ion (charged particles).
9u1.9 - The periodic table
The database of elements is called the Periodic Table. Here you can find all of the naturally occurring and artificial elements ordered byascending atomic number. The simplest element is Hydrogen with an atomic number of 1, and the highest element is the artificial element Oganneson with an atomic number of 118. The elements are usually represented by their symbols.

The AZE convention for representing atoms

9u1.10 - Metals and non-metals
Metals are a group of materials that share common properties
They are usually
- Shiny
- Hard
- Solid
- Malleable
- Ductile
- Good electrical conductors
- Good heat conductors
Notice, that it says "usually". This is because there are exceptions to all of these properties except one. All metals are good electrical conductors.
9u1.11 - Now test yourself
Click on the button below to access the self-tests for MYP9 and MYP10.