Unit 4 - We've got the power (Physical Science extension)
Content
Scheme of work
Evaporation and boiling
All liquids have some particles above the body of the liquid that have gained enough energy to escape from the inter-particular attractive forces.
These higher-energy particles form a gas. This gas is below the boiling point of the liquid and is called a vapour.
Definition:
A vapour is the gaseous form of a substance that exists below the boiling point.
The pressure exerted by the particles of vapour is dependent on the temperature. This pressure is known as the vapour pressure.
Experiment - Determine the rate of evaporation of some liquids
Students investigate propanone, ethanol and water
Experiment - The effect of a solute on rate of evaporation
Student design and implementation
Possible solutes: salt (sodium chloride), sugar (sucrose), copper(II) sulfate, potassium nitrate,
Chemical potential energy
Potential energy
Potential energy is a form of energy that can be converted into a more tangible form of energy. The gravitational potential energy of an object cannot be observed, but it can be calculated from the equation:
GPE = mgh
Where m = object mass, g = acceleration due to gravity (9.81 ms-2), h = height above the surface.
Example: Determine the gravitational potential energy of a wooden block of mass 0.2kg suspended 2 m above the floor
GPE = mgh
GPE = 0.2 x 9.81 x 2 = 3.924 J
Chemical potential energy
This is the energy that a substance has due to the nature of its bonds, with respect to all other substanced in the universe (yes, difficult).
The chemical potental energy can never be measured absolutely. However, it is possible to measure the change in chemical potential energy by converting some of it into heat and measuring the heat energy.
Here we are using the law of conservation of energy. Logically, the gain in heat energy must equal the loss in chemical potential energy, if not other energy is produced or work is done by the system (which uses energy).
The chemical potential energy that gets converted to heat (or vice versa) is know as the enthalpy change, represented by the symbol ΔH.
Calorimetry
This is the process of measurement of heat change in a system.
The change is temperature is a function of the change in heat energy, the mass and the capacity of the material to absorb heat - the specific heat capacity.
Most heat measurements take place in water or aqueous solution, which is mostly water. Water has a high capacity for heat absorbance - 4.18 kJ Kg °C-1.
This means that absorption of 4.18 kJ of energy by 1kg of water causes a change in temperature of exactly 1°C.
This can be expressed as a simple equation:
ΔE = mcΔT
The change in energy = the mass (in kg) x the specific heat capacity (4.18 kJ Kg °C) x the temperature change (in Celsius or Kelvin).
Apparatus used for calorimetry
A calorimeter is usually just a copper can, that may or may not be lagged (insulated) depending on the type of investigation.
Bond energy
The covalent bond
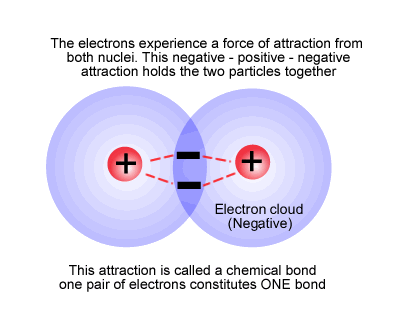
A covalent bond is a shared pair of electrons between two atoms in a molecule.
For example, chlorine gas consists of molecules, each of which contains two chlorine atoms joined together (bonded) by a single pair of electrons.
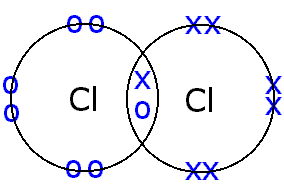
A covalent bond is formed, because the molecule is energetically more stable than the individual atoms.
Each chlorine outer shell now has a complete complement of eight electrons (an octet). This is a general case, as molecules are invariably more stable than the atoms that make them up.
Breaking the bond
To break the covalent bond we must apply energy, usually in the form of heat. This gives the individual atoms enough vibration to shake the molecule apart. The exact amount of energy needed to do this is called the bond energy. For chlorine the bond energy is equal to 242 kJ per mol of chlorine.
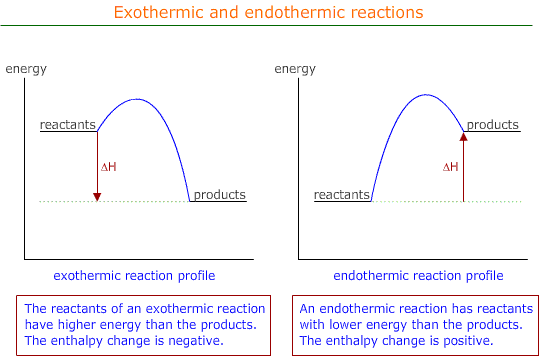
As the energy went into the atoms, the atoms gained energy and the energy change is said to be positive. This is always the case. Bond breaking requires energy, so bond breaking is an endothermic process and has a positive energy change.
The endothermic dissociation of chlorine
Cl2 → 2Cl· ΔH = +242 kJ mol-1
Bond formation
The law of conservation of energy states that "Energy can be neither created nor destroyed. It can only be transformed from one type to another."
A direct consequence of this law is the the reverse of any process involving energy must have an identical energy change but with the opposite sign.
Hence, the energy released when chlorine molecules are formed from chlorine atoms must be negative, i.e. the energy flow is from chemical potential energy to heat, and heat is given off.
Reactions in which heat is evolved are called exothermic processes.
The exothermic formation of chlorine bonds
2Cl· → Cl2 ΔH = -242 kJ mol-1
Graphical representation of energy changes
Greenhouse gases