Physical Science Enhancement - Chemistry 1 — Water
Content
- Chemistry u1.1 — Pipettes, burettes & uncertainty
- Chemistry u1.2 — Freezing/melting curve of water
- Chemistry u1.3 — Boiling point of water (Siwoloboff)
- Chemistry u1.4 — Density of water vs temperature
- Chemistry u1.5 — Solutions & volumetric flasks (molarity)
- Chemistry u1.6 — Dilution & solution properties
- Chemistry u1.7 — Water of crystallisation (CuSO4·5H2O)
Scheme of Work
Why water?
Ubiquitous in nature (~70% of Earth's surface; 60–70% of the human body).
Unusual physical properties: liquid at room temperature, high melting/boiling points, ice less dense than liquid.
Universal solvent for many substances; central to chemistry and life.
Chemistry u1.1 - The tools of chemistry
In these lessons you will become famiiar with the apparatus and instruments used in chemistry. You will also learn the techniques essential for investigation and experimentation.
You must learn the names of the specific pieces of apparatus, the usual dimensions (i.e. volumetric flasks have sizes ranging from 5 cm3 to 2000 cm3), and their correct use.
Pipettes, burettes & uncertainty
Deliver set volumes with different pipette sizes and from a burette; compare percentage uncertainties and discuss how instrument choice affects data quality.
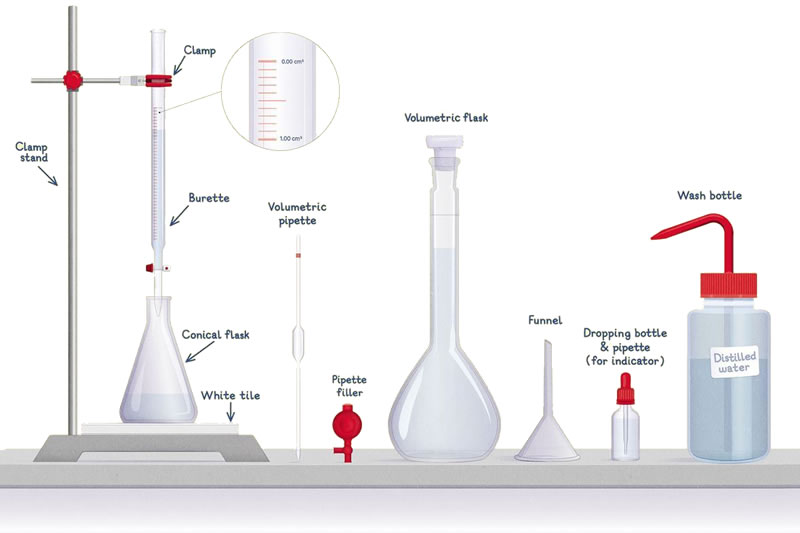
Activity - measuring volumes accurately
Apparatus
- 10.0 cm3 and 25.0 cm3 pipettes (5.00 cm3 if available), pipette filler.
- 50.0 cm3 burette + stand/clamp; balance (±0.01 g); beakers; wash bottle; distilled water.
Safety
- No mouth pipetting; keep bench/balance dry; handle glass carefully.
Core procedure (concise)
- Pipette 10.0 and 25.0 cm3 water into pre-weighed beakers; weigh to verify delivered mass.
- From a burette, deliver 10.0 and 25.0 cm3 into pre-weighed beakers; weigh.
- Record ambient temperature using a digital thermometer
- Use attached water density table to find the mass of 1.00 cm3 of water.
- Determine the percentage uncertainty for each delivery from instrument tolerances (written on the apparatus).
Estimating Uncertainty
Percentage uncertainty = (instrument tolerance ÷ reading) × 100.
If a 25 cm3 pipette has a tolerance of ± 0.16 cm3, then the percentage uncertainty in the volume that is delivered by the pipette = 100 x 0.16/25 = 0.64 %.
Hence we can state that the uncertainty in the volume delivered = ± 064%.
Add percentage contributions (volume ±, balance ±) for a quick combined %.
Convert to absolute uncertainty applied to the "answer", ensuring appropriate significant figures and precision.
Smaller delivered volumes give larger % for the same absolute tolerance.
Summary
- Instrument choice sets the floor for your measurement quality.
- Reporting with % uncertainty makes data comparable across groups.
Check your understanding
- Which delivery had the largest % uncertainty and why?
- How does using a 5.00 cm3 pipette instead of 25.0 cm3 affect % uncertainty?
- Why should the meniscus be read at eye level?
Chemistry u1.2 — Freezing/melting curve of water
Water has both interesting physical properties as well as chemical properties.
It has an unusually high melting and boiling point when compared to substances of similar relative mass. It also had the unusual property of a maximum density while liquid. This means that ice always forms at the surface of water, leaving warmer water below.
In this lesson you will produce a heating curve for crushed ice to water using a probe and loggerPro, data logging software.
Activity - The freezing point of water
Group setup & key equipment
- Crushed ice + splash of water; small beaker; temperature probe + data logger; hot plate or warm water bath.
Safety
- Care with hot plates and Bunsen burners.
Procedure
- The ice must be obtained from the freezer
- Insert probe into ice slurry; log temperature vs time through melting into liquid water.
- Identify the plateau region.
- Estimate the melting/freezing point of ice/water from the flat region.
Summary
- The flat region of the graphed data (plateau) is when the energy is being used to change the state at constant temperature.
Check your understanding
- What does the plateau reveal about energy changes at 0 °C?
- How could you reduce random scatter in your heating curve?
- Is melting a physical or chemical change? Explain briefly.
Chemistry u1.3 — Boiling point of water (Siwoloboff)
Determine the boiling point of water using the capillary (Siwoloboff) method in a glycerine bath.
Activity - Determine the boiling point of water
Group setup & key equipment
- Small test tube; Siwoloboff capillary (sealed end down); glycerine bath; thermometer/probe; hot plate; clamp/stand.
Safety
- Hot glycerine burns—heat slowly; avoid splashes; goggles on.
Core procedure (concise)
- Heat gently: record T when a continuous stream of bubbles appears.
- Cool: record T when bubbling just stops.
- Repeat until no further change in temperature.
Summary
- Boiling point depends on external pressure; lab results will differ from 100 °C.
- Alcobendas (Madrid) is at an altitude of about 700m above sea level. The "normal" atmospheric pressure in Madrid is usually about 94 kPa.
Check your understanding
- Why take both heating and cooling readings?
- How might a low-pressure weather system affect your measured Tb?
- Suggest one way to reduce uncertainty in this method.
Chemistry u1.4 — Density of water vs temperature
Measure density at different temperatures using a calibrated pipette and a balance; compare with literature data.
Student activity
Apparatus
- 10.0 cm3 pipette + filler; balance (±0.01 g); beakers; thermometer/probe; ice/warm water baths.
Safety
- Care with hot/cold water; standard PPE; avoid wet balances.
Procedure
- Tare container; deliver 10.0 cm3 water; weigh to get mass.
- Measure temperature; repeat at assigned temperatures (e.g., 10, 25, 40 °C).
- Compute density = mass ÷ volume; plot class data vs temperature.
Processing uncertainty
Calculate the percentage uncertainty of each step.
Add the percentage uncertainties to obtain the total percentage.
Reconvert percentage uncertainty into absolute uncertainty.
Summary
- Water’s density changes with temperature; measurements must be temperature-referenced.
- Adding % contributions approximates combined uncertainty for derived quantities.
Check your understanding
- Which measurement dominated your total % uncertainty, and why?
- Why must you report the temperature with your density value?
- Give one improvement to reduce uncertainty next time.
Chemistry u1.5 — Solutions & volumetric flasks (molarity)
Define solution, solute, solvent, and molarity; prepare a standard solution using a volumetric flask.
Student activity
Apparatus and chemicals
- Analytical/bench balance (±0.01 g); weighing boat/spatula; 100.0 cm3 volumetric flask + funnel; beaker; wash bottle; chosen solute.
Safety
- Avoid spills; clean bench; dry glassware; label all solutions clearly.
Core procedure
- You are required to prepare 100cm3 of 0.1 mol dm-3 copper(II) sulfate solution.
- Calculate required mass for target molarity and volume.
- Weigh solute; dissolve; transfer quantitatively; make to the mark; invert to mix thoroughly.
- Label with substance, concentration, date, initials.
Determine the uncertainty in your solution concentration by calculating the inaccuracy percentage at each step of the preparation.
How could you determine the actual concentration of the prepared solution?
The solution should be saved for the next activity.
Summary
- Accurate solution preparation depends on careful weighing and making to volume.
- Concentration should be reported with an uncertainty estimate.
Check your understanding
- Define solute, solvent, solution, and molarity in one sentence each.
- Where could systematic error arise during solution preparation?
- Why invert a volumetric flask several times after making to the mark?
Chemistry u1.6 — Dilution & solution properties
Dilution is a standard method of preparing solutions of lower concentration from ones of higher concentration.
Dilution involves measuring out an accurate volume of the stock solution, transferring it to a volumetric flask and making up to the mark with water, ideally, distilled or deionized water should be used.
Students often try to measure out unfeasible volumes of solution rather than carry out easy dilutions. It is important to understand that science does not require whole numbers!
Example: Diluting by a factor of 10
A 25cm3 aliquot (portion) of the stock solution is pipetted into a 250 volumetric flask.
The volumetric flask is then filled to the mark with distilled or deionized water.
The volumetric flask is inverted several times to mix the contents thoroughly and labelled with the new concentration.
The amount of solute is determined using the relationship:
mol = concentration x volume (dm3)
Activity: Dilution of a stock solution
Apparatus and chemicals
- Stock standard solution (the copper(II) sulfate solution prepared in the previous activity); pipettes (10ml, 25ml, 50ml); volumetric flasks (100ml, 250ml); balance; beakers; conductivity apparatus.
Safety
- Standard PPE
- Careful handling of glassware
- Copper(II) sulfate is toxic.
Procedure
- Plan a dilution series (each group targets a different concentration).
- Prepare dilutions; measure chosen property (e.g., density) for each.
- Determine the absolute inaccuracy of each solution.
Summary
- Dilution planning links calculation to practical volume handling.
- Trends across pooled data strengthen conclusions.
Check your understanding
- Rearrange C1V1 = C2V2 to solve for V1.
- Which sources of uncertainty affect the final concentration the most?
- Did your conductivity–concentration trend fit your prediction? Give one reason if not.
Chemistry u1.7 — Water of crystallisation (CuSO4·5H2O)
Prepare copper(II) sulfate solution from acid and excess copper(II) oxide; hot-filter, then crystallise; compare actual vs theoretical yield; observe dehydration/rehydration as a reversible change.
Activity - Preparation of copper(II) sulfate crystals by chemical reaction
Apparatus and chemicals
- 1.00 mol dm−3 H2SO4; CuO powder (excess); hot plate; filtration setup; evaporating basin/watch glass; tongs.
Safety
- H2SO4 is corrosive; CuSO4/CuO harmful—gloves & goggles required.
- Hot solutions and glassware—handle with tongs; heat gently.
- Dispose of copper-containing waste per school policy.
Procedure
- Pipette 25.0 cm3 of acid; warm and add CuO to (slight) excess until a little remains.
- Hot-filter into a weighed evaporating basin to remove unreacted solid.
- Evaporate filtrate to crystallise - this may take a couple of days.
- Dry and re-weigh.
- Determine the mass of copper(II) sulfate prepared.
Example:
If 25cm3 of 1 mol dm-3 sulfuric acid is used with excess copper(II) oxide then the moles of sulfuric acid = 1 x 0.025 = 0.025 mol
Theoretical mol of copper(II) sulfate = 0.025 mol
The relative mass of copper(II) sulfate, CuSO4 = 159.5
The theoretical mass of copper(II) sulfate that can be formed = mol x relative mass = 0.025 x 159.5 = 3.99 g
Summary
- Hot filtration and controlled evaporation produce hydrated crystals.
- Yield analysis links practical work to quantitative reasoning.
Check your understanding
- Identify two steps most likely to reduce yield and explain why.
- What observation shows dehydration and rehydration of copper(II) sulfate?
- Why use a slight excess of CuO rather than excess acid?
Activity - Determining the formula of hydrated copper(II) sulfate
Apparatus and chemicals
- Hydrated copper(II) sulfate crystals
- Nickel crucible and crucible tongs
- Bunsen burner, tripod, pipeclay triangle, heat-proof mat
- Electronic balance with heat proof cover
Safety
- The crucible will be very hot - care
- Students must tie long hair back and wear safety glasses at all times
- The hot crucible must only be set down on heat-proof surfaces
Procedure
- Weigh the nickel crucible
- Using a spatula, add approximately 1g of hydrated copper(II) sulfate crystals
- Heat the crucible using a transparent Bunsen flame
- Remove from time to time and reweigh until there is no further mass change
The mass loss is due to the water of crystallisation.
The mol of water lost = mass loss/18 (relative mass of water)
The mol of copper(II) sulfate is the mass of the residue/159.5
Use these two values to find out the ratio of copper(II) sulfate to water in the hydrated crystals. This can be expressed in the form: CuSO4·xH2O