Unit 8 - Food for Thought
Content
- MYP10u8.1 - Acids and bases
- MYP10u8.2 - The pH scale
- MYP10u8.3 - Indicators
- MYP10u8.4 - Neutralisation
- MYP10u8.5 - Food preservation and spoiling
- MYP10u8.6 - Germ theory
- MYP10u8.7 - Polymers, natural and artificial packaging
- MYP10u8.8 - The structure of DNA
- MYP10u8.9 - Genetically modified organisms
- MYP10u8.10 - Now test yourself
Scheme of Work
10u8.1 - Acids and bases
This unit is all about the science of food. To begin, we need to understand the chemistry of substances we eat and use every day. In this lesson, we explore acids and bases — two types of substances that are found in foods, drinks, cleaning products, and even in your body.
What are acids and bases?
Acids and bases are groups of chemicals with opposite properties. Acids usually taste sour and can react with metals. Bases often feel slippery and are found in soaps and cleaning products. When mixed, acids and bases can neutralise each other.
- Acids release hydrogen ions (H⁺) in solution.
- Bases release hydroxide ions (OH⁻) in solution.
Example
Lemon juice contains citric acid — that’s why it tastes sharp and sour. Soap feels slippery because it contains a base like sodium hydroxide. Vinegar, which contains ethanoic acid, is another common acid found in food. Ant stings contain methanoic acid, very similar to ethanoic acid only stronger.
Ethanoic acid - CH3COOH
Methanoic acid - HCOOH
Common examples:
- Acids: citrus fruits, vinegar, fizzy drinks, stomach acid
- Bases: soap, toothpaste, baking soda, drain cleaner

Common acids and bases
Many fruits contain weak acids, although citrus fruits (oranges, lemons, grapefruit and limes) are perhaps the most commonly known examples. They contain both citric acid and ascorbic acid (vitamin C).
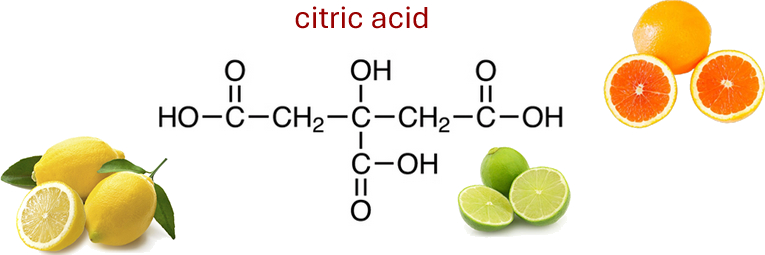
Grapes contain tartaric acid and apples contain maleic acid.
Activity: Acid and base hunt
Look around your home and find three acids and three bases (check labels and ingredients). Make a table with their names, what they're used for, and whether they are safe to taste or touch. Always ask an adult before handling unfamiliar substances!
Summary
- Acids and bases are common substances with opposite properties.
- Acids release hydrogen ions (H⁺); bases release hydroxide ions (OH⁻).
- They are found in food, drinks, cleaning products, and even inside our bodies.

Lemons and soap: everyday examples of acids and bases
Check your understanding
- What’s the difference between an acid and a base?
- Give two examples of each from your kitchen or bathroom.
10u8.2 - The pH scale
In section 8.1, we learned about acids and bases — two types of chemicals that behave very differently. But how can we tell how strong an acid or base is? In this lesson, we explore the pH scale, a tool that helps us measure how acidic or basic a substance is.
What is the pH scale?
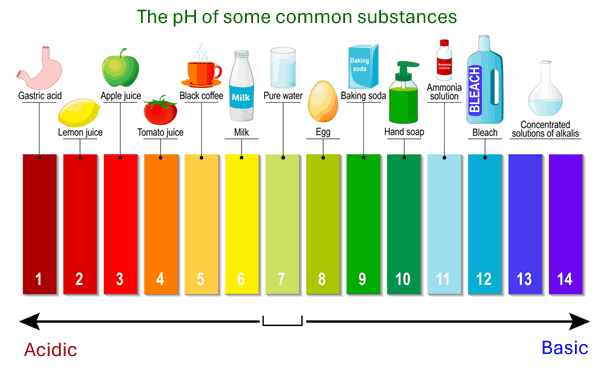
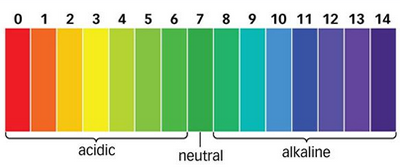
The pH scale runs from 0 to 14 and tells us how acidic or basic a solution is. A lower pH means a stronger acid, while a higher pH means a stronger base. A pH of 7 is neutral — not acidic or basic.
- pH 0–6: Acidic (e.g. lemon juice, vinegar)
- pH 7: Neutral (e.g. pure water)
- pH 8–14: Basic (e.g. baking soda, soap)
Example
Vinegar has a pH of around 3, making it a weak acid. Baking soda has a pH of about 9, so it’s a weak base. Pure water, with a pH of 7, is perfectly neutral.
How is pH measured?
We can measure pH in several ways:
- Indicator paper – turns red in acid and blue in base (simple but limited)
- Universal indicator – changes colour across the full pH scale
- pH meters – electronic devices that give a numerical value
Activity: Test household substances
Use universal indicator paper (or a pH test kit) to test the pH of safe household substances like lemon juice, milk, soapy water, or fizzy drinks.
Record the pH and organise your results in a table from most acidic to most basic.
Summary
- The pH scale ranges from 0 to 14 and measures acidity or basicity.
- Acids have low pH, bases have high pH, and neutral substances are at pH 7.
- We can measure pH using indicators or digital meters.
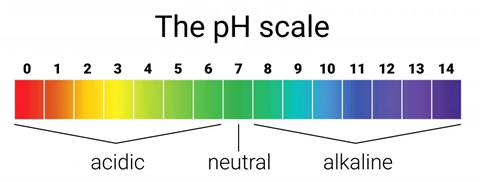
The pH scale helps us classify substances from acidic to basic
Check your understanding
- What pH values represent acids, bases, and neutral substances?
- What are two ways we can measure the pH of a solution?
10u8.3 - Indicators
In Lesson 2, we learned how the pH scale measures how acidic or basic a substance is. But how do we actually observe pH? In this lesson, we explore indicators — special substances that change colour depending on the pH of a solution. They help us see the chemistry in action.
What are indicators?
Indicators are chemicals that change colour when added to a solution. The colour tells us whether the substance is acidic, neutral, or basic. Some indicators show a clear yes/no result, while others show a range of colours depending on the pH.
Example
Litmus paper turns red in acids and blue in bases. Universal indicator shows a full colour spectrum from red (pH 1) to purple (pH 14), giving a more detailed idea of the pH value.
Common types of indicators:
- Litmus paper – red in acid, blue in base, no colour change in neutral
- Universal indicator – shows a range of colours for different pH values
- Natural indicators – like red cabbage juice, turmeric, and beetroot extract
Why use indicators?
Indicators are quick, easy, and safe ways to classify substances. They're useful in chemistry labs, schools, environmental testing, and even food science. Some are sensitive enough to detect very small changes in pH.
Universal indicator showing colours across the full pH range
Activity: Make a red cabbage pH scale
Boil red cabbage in water to extract the cabbage juice. Filter it into small glass beakers and add small amounts of different household substances (e.g. vinegar, baking soda, lemon juice, shampoo, saltwater).
Observe the colour change and match it to approximate pH values.
Summary
- Indicators show whether a substance is acidic, basic, or neutral using colour changes.
- Universal indicator gives a full pH colour scale; litmus paper gives a simple acid/base result.
- Natural indicators can be made from plants like red cabbage.
Check your understanding
- What does an indicator do?
- How is universal indicator more useful than litmus paper?
- Name one natural indicator you could make at home.
10u8.4 - Neutralisation
In the last lesson, we used indicators to detect whether a solution is acidic or basic. In this lesson, we explore what happens when an acid and a base are mixed together. This chemical reaction is called neutralisation, and it has many useful applications in everyday life.
What is neutralisation?
Neutralisation is a chemical reaction between an acid and a base. When they react, they cancel each other out, forming a salt and water. This brings the pH closer to 7 - neutral.
This is one of the most common chemical reactions and you should learn it!
Acid + Base → Salt + Water
Neutralisation reactions
Example
Hydrochloric acid (HCl) reacts with sodium hydroxide (NaOH) to form sodium chloride (NaCl) — table salt — and water.
HCl + NaOH → NaCl + H2O
Example
Nitric acid (HNO3) reacts with sodium hydroxide (NaOH) to form sodium nitrate (NaNO3) and water.
HNO3 + NaOH → NaNO3 + H2O
Why is neutralisation useful?
- It treats acidic soil in farming (lime is added to neutralise the pH).
- It relieves indigestion (antacids neutralise stomach acid).
- It cleans up chemical spills (bases neutralise acid spills and vice versa).
These reactions are often associated with release of energy in the form of heat. They allow us to prepare (synthesise) salts in the laboratory, by chosing appropriate acids and bases.
Synthesis of salts
Example
To prepare a sample of sodium ethanoate we must choose the appropriate acid and base. Ethanoates are prepared from ethanoic acid. Sodium salts may be prepared using the base sodium hydroxide (although this is not the only choice, sodium carbonate could also be used).
CH3COOH + NaOH → CH3COONa + H2O
Example
To prepare a sample of potassium nitrate we must choose the appropriate acid and base. Nitrates are prepared from nitric acid. Potassium salts may be prepared using the base potassium hydroxide (although this is not the only base, we could also use potassium carbonate) .
Nitric acid (HNO3) reacts with potassium hydroxide (KOH) forming potassium nitrate (KNO3) and water.
HNO3 + KOH → KNO3 + H2O

Antacids neutralise stomach acid to reduce discomfort
Activity: Neutralise vinegar with baking soda
Slowly add baking soda to vinegar in a cup. Use indicator paper or red cabbage juice to watch the pH change. Does the bubbling stop when neutralisation is complete? What colour shows neutrality?
Summary
- Neutralisation is a reaction between an acid and a base to form salt and water.
- This process moves the pH toward 7 (neutral).
- Neutralisation is useful in medicine, agriculture, and chemical safety.
Check your understanding
- What are the products of a neutralisation reaction?
- How does pH change during neutralisation?
- Give one example of how neutralisation is used in daily life.
10u8.5 - Food preservation and spoiling
In the last lesson, we saw how acids and bases can cancel each other out in a process called neutralisation. This has real-life applications in food safety, such as pickling. In this lesson, we look at what causes food to spoil and how we can prevent it using chemical and biological understanding.
What causes food to spoil?
Food spoils because of the action of microbes, enzymes, and chemical reactions such as oxidation. These changes can affect taste, smell, texture, and even make food unsafe to eat.
- Bacteria and fungi multiply on food, especially when it’s warm and moist.
- Enzymes inside food cause ripening and eventually breakdown.
- Oxygen can react with food, causing colour and flavour changes.
Example
Milk goes sour when bacteria break down lactose into lactic acid. Bananas turn brown due to enzymes and oxidation. Bread can grow mould in warm, humid conditions.
How can we preserve food?
Food preservation methods aim to slow or stop the processes that lead to spoilage. This is done by controlling temperature, moisture, pH, or oxygen.
- Refrigeration slows down microbial growth.
- Freezing stops microbes and enzyme activity almost completely.
- Drying removes water that microbes need to survive.
- Pickling lowers the pH to prevent microbial growth.
- Vacuum packing removes oxygen to prevent spoilage reactions.
Example
Jerky is preserved by drying and salting. Pickled onions are stored in acidic vinegar. Vacuum-packed cheese stays fresh longer by keeping air out.

Examples of preserved foods using drying, pickling, and vacuum sealing
Research activity: Investigate food expiry labels
Look at different food packages at home. What preservation methods are used? What’s the difference between “best before” and “use by”? How does packaging design help prevent spoilage?
Summary
- Food spoils because of microbial activity, enzymes, and oxidation.
- Preservation methods slow spoilage by changing conditions like temperature, pH, or oxygen.
- Common methods include refrigeration, drying, pickling, and vacuum sealing.
Check your understanding
- What are three causes of food spoilage?
- List two methods of food preservation and explain how they work.
10u8.6 - Germ theory
In the last lesson, we saw how food can spoil because of microorganisms like bacteria and fungi. But how do we know these tiny organisms exist, and what role do they play in disease and decay? This lesson introduces germ theory — the idea that many diseases and spoilage processes are caused by microscopic life forms.
What is germ theory?
Germ theory is the scientific concept that certain diseases and food spoilage are caused by microorganisms such as bacteria, viruses, and fungi. These microbes are too small to see with the naked eye, but they can multiply quickly and produce harmful effects in living things and in food.
Before germ theory was accepted, people believed that bad air or supernatural forces caused illness. Scientists like Louis Pasteur and Robert Koch changed this by showing that specific microbes cause specific diseases and that food spoils due to microbial activity.
Example
Pasteur demonstrated that broth stayed clear if no microbes entered it, but spoiled when exposed to air.
Koch grew bacteria in a lab and proved they caused diseases like tuberculosis by infecting animals with isolated strains.
How do microbes spread?
- Through the air (e.g. coughing, sneezing)
- By touching contaminated surfaces
- Through food or water (e.g. spoiled food, untreated water)
- By insects, animals, or unwashed hands
Why this matters for food safety:
Understanding germ theory helps us protect food and prevent illness. We can avoid contamination by washing hands, cooking food thoroughly, and storing it properly to limit microbial growth. Many preservation methods are designed to prevent germs from spreading or surviving.

Louis Pasteur helped prove that microbes cause food spoilage and disease
Experiment: How clean are your hands?
Press a finger on a slice of bread before and after washing your hands. Seal the slices in bags and observe them over several days.
Compare the growth of mould or other changes. Make sure the bags stay closed and observe from the outside only.
Summary
- Germ theory explains that microbes cause many diseases and food spoilage.
- Key scientists like Pasteur and Koch provided evidence for germ theory.
- Microbes spread through air, surfaces, food, water, and contact.
- Hygiene and proper food handling are essential for controlling microbes.
Check your understanding
- Who were two key scientists that helped develop germ theory, and what did each of them do?
- How does germ theory help explain food spoilage?
- What practices can you follow at home to prevent food contamination?
10u8.7 - Polymers, natural and artificial packaging
In the last lesson, we explored how microbes cause food spoilage and the importance of hygiene. But even with careful handling, how we package food plays a key role in keeping it fresh. In this lesson, you’ll learn about polymers — long-chain molecules used in natural and artificial materials — and how they're used to package and preserve food.
What are polymers?
Polymers are large molecules made from repeating units called monomers. Some polymers occur naturally, like cellulose in plants or proteins in animals. Others are synthetic (man-made), like plastics used in packaging.
Example
Plastic bottles are made from a polymer called PET (polyethylene terephthalate). Natural polymers like starch can also be processed into biodegradable films for food wrap.
Why are polymers used in packaging?
Polymers are lightweight, flexible, and can form airtight or watertight barriers — perfect for keeping food safe from moisture, microbes, and oxygen. Some plastics are transparent so consumers can see the food inside, while others block light to slow spoilage.
- Plastic containers keep food sealed from bacteria and air.
- Vacuum-sealed polymer bags remove oxygen and reduce oxidation.
- Cling film wraps tightly around food to prevent contamination.
Example
A chocolate bar is often wrapped in multiple polymer layers: one to keep out moisture, one to block light, and one for branding. Yogurt pots are made of plastic for strength and a foil seal to keep the contents fresh.
Environmental impact:
Many synthetic polymers (like plastics) do not break down easily. They can stay in the environment for hundreds of years. To reduce waste, scientists are developing biodegradable polymers made from renewable materials like corn starch or algae.
Natural and synthetic polymers

Activity: Compare packaging types
Collect a few different types of food packaging (plastic wrap, cardboard box, foil pouch). Identify whether the material is a polymer and whether it’s natural, synthetic, or biodegradable.
Find the kite mark on the synthetic polymers and research its details.
Create a chart showing which packaging is best for keeping food fresh and for the environment.
Summary
- Polymers are large molecules used in many types of packaging.
- They help protect food from microbes, air, and moisture.
- Packaging polymers can be natural (like starch) or synthetic (like plastic).
- Biodegradable polymers offer a more sustainable alternative to traditional plastics.
Check your understanding
- What is a polymer, and why are polymers useful in food packaging?
- What are two problems caused by synthetic polymers in the environment?
10u8.8 - DNA
In the last lesson, we looked at the materials used to preserve food and reduce spoilage. Now we zoom in to the microscopic level to explore DNA — the molecule that carries instructions for life. DNA helps determine the traits of all living things, from how organisms grow to how they respond to diseases and environments.
What is DNA?
DNA stands for deoxyribonucleic acid. It is found in the nucleus of most cells and contains the instructions an organism needs to develop, function, and reproduce. DNA is made up of two strands twisted into a double helix — like a twisted ladder.
Example
The genes in your DNA determine your eye colour, blood type, and even how your body processes nutrients. In plants, DNA controls how fast fruit ripens or how big leaves grow.
Structure of DNA:
The sides of the DNA "ladder" are made of sugar and phosphate molecules. The "rungs" are pairs of chemical bases: A (adenine) pairs with T (thymine), and C (cytosine) pairs with G (guanine). This base pairing is what allows DNA to be copied exactly during cell division.
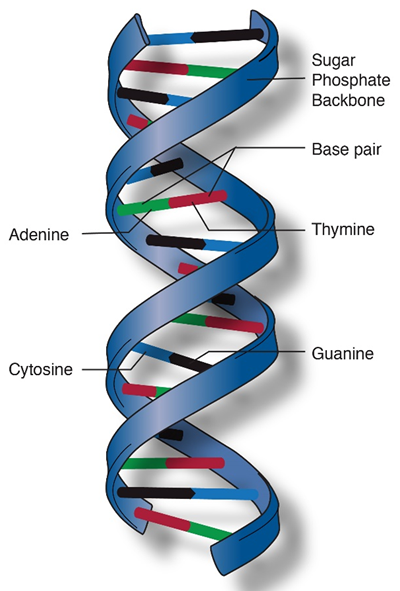
The DNA double helix and base pairings
Why is DNA important?
DNA is like a blueprint. It tells each cell what proteins to make and when. If DNA is changed (mutated), the instructions can be altered, which may lead to new traits — helpful, harmful, or neutral. This is the basis of genetic engineering, which we’ll explore in the next lesson.
Activity: Model the DNA helix
Use coloured paper, sweets, or pipe cleaners to build a physical model of DNA. Show the backbone and base pairs using different colours or shapes. Label the parts and identify which base pairs with which.
Summary
- DNA carries instructions for all living organisms.
- It has a double-helix structure made of sugar-phosphate sides and base pair rungs.
- DNA controls how organisms grow, look, and function.
- Mutations in DNA can lead to new traits and are important in biotechnology.
Check your understanding
- What are the four chemical bases in DNA and how do they pair?
- Why is DNA important to how an organism functions?
10u8.9 - Genetically modified organisms
In the last lesson, you learned that DNA carries the instructions for life. Scientists have discovered how to read and even edit these instructions. When we change an organism's DNA to give it new traits, we create what’s known as a genetically modified organism (GMO).
What is a GMO?
A GMO is any living organism whose genetic material has been altered using science. This could be done by inserting a gene from one organism into another, turning off certain genes, or editing DNA to give it new functions.
Example
Scientists can add a gene from bacteria to a plant, making it resistant to insect pests. The plant produces its own natural protection without needing chemical sprays.
Why do we use GMOs?
- To increase crop yields
- To make food last longer on shelves
- To improve nutrition (e.g. golden rice with vitamin A)
- To reduce the need for pesticides
Are GMOs safe?
There is ongoing debate about the safety and ethics of GMOs. Most scientific studies show that approved GMOs are safe to eat, but some people prefer organic or non-GMO foods due to environmental or health concerns. GMOs are carefully tested before being used in food.
Activity: Investigate GMO food labelling
Look at food packages in your home or local shop. Can you find any labels that say “GMO” or “non-GMO”? Research what rules exist in your country about labelling genetically modified food. Should consumers have a choice?
Summary
- GMOs are organisms with altered DNA to give them new traits.
- They are used in agriculture to improve food production, nutrition, and sustainability.
- GMO safety and ethics are debated, but they are widely tested and regulated.
Check your understanding
- What is a GMO, and how is it created?
- List two reasons why scientists create genetically modified plants.
- What are some concerns people may have about GMOs?
10u8.10 - Now test yourself
Click on the button below to access the self-tests for MYP9 and MYP10.