Physical Science Enhancement - Chemistry 5 - Metals and Redox
Introduction
In the periodic table the majority of elements are metals. A metal is a substance that has metallic properties:
The only property that ALL metals exhibit is electrical conductivity. This is because they have outer electrons that are only loosely held to the nucleus.
Content
Scheme of work
C5.1 Metallic character
Metallic character is exemplified by the properties that are typical of metals. Metals have loosely held outer (valence) electrons. The more reactive the metal, the more easily it can lose these outer electrons forming a positive ion.
Group 1 metals are very reactive precisely for this reason - their atoms are much more stable as an ion than as a metal.
Na → Na+ + 1e-
The oxidation of sodium by removal of an electron
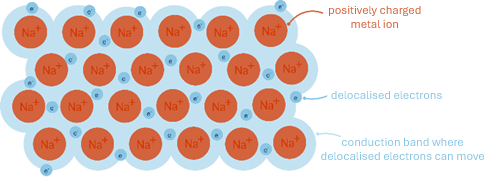
All metals conduct electricity, as the metal structure has a regular lattice of metal ions surrounded by a “sea” of delocalised electrons. This is the most important physical property of metals and applies to ALL metals.
Metals are usually:
- Hard
- Malleable
- Ductile
- Shiny
- Good conductors of heat
- High melting point solids
Experiment C5.11 - The properties of metals
1. Plot a graph of different types of wire - length against mass hung on the wire. What are the variables?
Different metals have different ductility. Why do you think this is?
2. Measure the current that passes through a length of different metal wires (very low voltage setting (2V). What are the variables?
Ohms's law
Voltage (volts) = Current (amps) x Resistance (Ohms)
V=IR
3. Data research the melting points of some metals.
- iron
- zinc
- magnesium
- aluminium
- copper
- sodium
- potassium
- mercury
How do you think this information should be presented?
What does this show us?*
Teacher guidance
Explain these properties using the microscopic arrangement of particles. The forces that hold the particles (ions) together are non-directional.
The regular lattice allows the particles to slide over one another (malleable and ductile) and the non-directional forces keep the particles (ions) together.
That there is weak bonding in group 1, but also that are exceptions. Exceptions are due to some elements having a tendency towards covalent bonding within the metallic structure (making loosely held molecules).
Discuss with the students – chemistry often has “grey areas” where an element is not really a typical metal, but displays characteristics of non-metals also. This happens in the boundary between metals and non-metals. Hence, lead, tin, gallium etc have very low mp.
Electricity
Differentiate between voltage (the push) and current.
Explain current as a flow of electrical charge. In this case carried by electrons.
Each electron carries an electrical charge in Coulombs (-1.6 x 10-19C)
1 Coulomb of charge flowing per second = 1 Amp
Amps = Coulombs/time(s)
Conventional and real current
In physics, for historical reasons the electrical current traditionally moves from positive to negative terminals of the power supply. This was a convention agreed on by physicists in the 19th Century. For this reason, it is called conventional current.
However, in chemistry we know that electrons carry a negative charge, so the actual current moves from negative to positive terminals.
Definitions
- Delocalised – not having a specific location
- Lattice – regular arrangement of repeating particles
- Malleable – can be moulded into shape
- Ductile – can be drawn into wires
C5.2 - Displacement reactions
More reactive metals can transfer their valence electrons to the ions of less reactive metals. This is called a redox reaction, where one species loses electron(s) and another species gains the electron(s).
Ionic compounds dissociate 100% in solution. This is how/why water dissolves ionic compounds.
CuSO4(s) + (aq) → Cu2+(aq) + SO42-(aq)
In all ionic solutions, the individual ions are free to move independently. In other words, copper(II) sulfate solution contains water, copper ions and sulfate ions all mixed together and moving independently.
Experiment C5.21 - Investigate metals and solutions containing dissolved metal ions.
In the following activity lesson you will carry out micro-scale displacement reactions to build a reactivity series for common metals. You will record observations and write three versions of each equation: full equation, total ionic, and net ionic. You will also identify spectator ions — ions that appear unchanged on both sides of the equation.
Available Metals: Iron, copper, zinc, magnesium, lead
Metal salts in solution: Iron(II) sulfate, copper(II) sulfate, zinc sulfate, magnesium sulfate, lead nitrate.
Teaching notes
The students use spotting plates with small squares of metal. The students should produce ionic equations for any reactions that they observe.
More reactive metals displace less reactive metals from solutions containing their ions
copper ions + zinc metal → zinc ions + copper metal
This is an electron transfer reaction. It is also exothermic (releases heat to the surroundings). It is an example of a REDOX (reduction and oxidation) reaction. The zinc atoms transfer two outer electrons to the copper ions and in doing so become ions themselves.
Zn(s) + Cu2+(aq) → Cu(s) + Zn2+(aq)
Zinc dissolves as the colour of blue copper(II) aqueous ions fades and the copper metal is deposited as a brownish mass.
- Zinc is oxidised by copper ions.
- Copper ions are reduced to copper metal.
- The reducing AGENT is the zinc
- The oxidising AGENT is the copper ions.
Note: An AGENT is something that causes an event to occur
REDOX stands for reduction and oxidation. Reduction is gain of electrons and oxidation is loss of electrons. An easy way to remember this is to use the mnemonic OILRIG.

Oxidation Is Loss, Reduction Is Gain
- Definitions
- Oxidation is loss of electrons (by a species)
- Reduction is gain of electrons (by a species)
- Spectator ions: Ions present in a solution that do not take part in the reaction
- Mnemonic: a memory aid
Experiment C5.22 - Investigating the energy released when zinc metal reduces copper ions.
- Chemicals
- Copper(II) sulfate, 1.0 mol dm-3
- Zinc powder
- Apparatus
- Polystyrene beaker
- Glass beaker, 250 cm3
- Thermometer
- Pipette, 25 cm3, and filler
Instructions
Pipette 25 cm3 of copper(II) sulfate solution into a weighed polystyrene beaker and reweigh.
Measure approximately 2.5 g of zinc powder (an excess)
Record the temperature of the copper(II) sulfate solution.
Start the timer and record the temperature (thermometer or temperature probe)
After 30 seconds stir the zinc powder into the copper(II) sulfate solution using the thermometer.
Keep stirring and record the temperature every minute for 10 minutes.
Dispose of the mixture in a waste container, provided by your teacher.
Repeat the procedure twice more.
C5.3 Corrosion
Corrosion – Literally “chemical erosion”, breaking something down by chemical attack. Corrosion could be described as wearing down by chemical action, if you like, chemical erosion. The waves break down the rocks on the shore by mechanical erosion, but a process that wears something down by chemical attack and reaction is corrosion.
Corrosion quietly destroys structures and costs huge amounts of money each year.
Corrosion is an electrochemical process: the metal is oxidised (loses electrons), often by dissolved oxygen in water. On iron in aerated water: anode and cathode spots form on the same surface or between dissimilar metals.
Starter: why do structures fail?
- Watch a short video about a historical bridge failure; discuss how tiny defects + corrosion can lead to catastrophic failure.
- Brainstorm: where do you see corrosion in daily life? (bikes, boats, cars, outdoor fixtures)
Economics of corrosion
A widely-cited estimate from the National Association of Corrosion Engineers (NACE) puts the annual global cost of corrosion at about US $2.5 trillion, which is roughly 3–4 % of world GDP.
Some more recent industry reports suggest the total might now be over $3 trillion annually, depending on the methodology.
The mechanism of corrosion
Corrosion is degradation caused by the reaction of metals with something in the air, usually oxygen. The most important and widely used construction metal is iron. Iron corrodes in air, particularly in the presence of air and water.
Electrons are transferred from iron to oxygen molecules. Solutions containing ions, which can carry charge, help this process along.
Iron reacts with oxygen in the presence of water to form hydrated iron(III) oxide (rust). The overall equation is:
4Fe(s) + 2xH2O(l) + 3O2(g) → 2Fe2O3.xH2O(s)
We can break this equation down into just the oxidation and reduction parts (half-equations)
Oxidation: Fe → Fe3+ + 3e-
Reduction: O2 + 4e- → 2O2-
The balanced ionic equation is obtained by equalising the electrons and then adding them together.
4Fe + 3O2 → 4Fe3+ + 6O2-
Demonstration: The oxidation of iron
The oxidation of an iron nail using dilute sulfuric acid in the presence of hydrogen peroxide and potassium thiocyanate (petri dish + projector).
This models the rusting process by reacting iron with hydrogen ions in the presence of an oxidising agent. Potassium thiocyanate shows up the iron(II) ions by producing a dark red coloured complex.
Factors affecting rusting
Cars are observed to rust more rapidly near to the seaside.
Propose a research question and hypothesis to explain this observation.
Plan a method to test the hypothesis. The variables MUST be quantitative.
- Available materials
- Small iron rods
- Iron nails large and small
- Iron filings (powder)
- Sodium chloride, common salt/sea salt
- Deionised water, boiled water, tap water
- Zinc strips
- All the usual laboratory apparatus and instruments
Check your understanding
- Explain, with half-equations, why an iron nail wrapped with copper corrodes faster in salt water.
- Why does zinc protect iron when they are in contact? Include the anode and cathode half-equations.
- Which method combats corrosion by removing the electrolyte: paint, sacrificial anode, or stainless steel? Justify.
C5.4 - Reduction of metal oxides
In the previous section we have seen that metals corrode by reacting with the oxygen, water and carbon dioxide in the air. The products of corrosion do not have the required metallic characteristics and so are not welcome.
Chemists extract metals from their oxides, reversing the process of oxidation.
Metal oxides can be reduced to the pure metal by using a reducing AGENT, which is a substance that is better at releasing electrons then the metal itself.
Copper(II) oxide can be reduced by heating in a stream of hydrogen gas or methane gas.
CuO + H2 → Cu + H2O
Teaching notes
This is an interactive virtual experiment to determine the formula of the metal oxide using computer generated data. However, should the teacher so decide it is practical to carry out in the laboratory using copper(II) oxide.
Safety
Care should be taken to flush the test tube with gas before igniting it at the exit hole. If the laboratory is quiet, the gas can be heard passing through the small exit hole.
After the reduction is complete the heating should be stopped and the Bunsen burner turned off, but the flow of gas must continue until the test-tube is cooled down to ensure that oxygen does not enter the test-tube and re-oxidise the hot metal.
Iron and steel
Industrially iron, which is the most important construction metal in modern society, is extracted from its ore by reduction with carbon monoxide, which in turn is formed by heating coke (dried coal) in a reduced supply of air.
Iron is extracted from various iron oxides by heating with carbon monoxide, formed from either charcoal (small scale) or coke (dried coal) burning in limited air supply.
The Blast furnace extraction of iron
The thermit reaction
On a small-scale, iron can be extracted rapidly by reducing iron(III) oxide using aluminium as the reducing agent. This is known as the thermit reaction.
The Thermit Reaction
DEMO Thermit
This must be carried out in the open air or in a fume hood that has previously been emptied.
Aluminium reduces iron(III) oxide in a highly exothermic reaction to produce pure iron.
2Al(s) + Fe2O3(s) → Al2O3(s) + 2Fe(l)
Teaching notes
Optional activity
Compare and contrast - Thermite & Blast furnace methods for making iron.
Similarities
Both exothermic
Both reduce iron(III) oxide to iron
Both require initiation
Both make molten iron
Differences
Thermit is small scale, blast furnace large scale
Thermit is one-off, blast furnace is continuous
Thermit uses expensive alumnium as the reducing agent. blast furnace uses carbon/carbon monoxide.
Thermit makes pure iron, blast furnace makes iron/carbon mixture
Thermit waste products leave as smoke, blast furnace waste run off as liquid slag
C5.5 - Voltaic Cell
Voltaic or Galvanic cells are constructed to generate an electrical potential from chemical energy. The chemical energy is converted into electrical energy that can be made to drive a current around an electrical circuit.
In this process we are taking advantage of the different reactivity of metals and metal ion solutions. The more reactive metal passes electrons to the ions of the less reactive metal using the external circuit.
The difference in reactivity between the metals in each half-cell results in the chemical potential energy. This causes electrons to attempt to move from one substance to another (a redox reaction). If the electrons are allowed to flow around the external circuit a flow of electrical charge occurs. This is known as an electrical current.
Investigation - Cell construction and measurement of cell potential
Assemble a zinc–copper galvanic cell and explain how it works using full, total ionic, and net ionic equations.
- Electrodes: zinc strip and copper strip (cleaned with sandpaper).
- Solutions: 0.1 M ZnSO4 and 0.1 M CuSO4 in separate beakers.
- Salt bridge: filter paper strip soaked in 1.0 M KNO3. Potassium and nitrate ions flow in the salt bridge to balance charge as current flows.
- Leads and multimeter. Optional small load: LED with a series resistor (≥220 Ω) or a low-current buzzer.
Procedure
- Place Zn in ZnSO4(aq) and Cu in CuSO4(aq).
- Connect the two solutions with the salt bridge.
- Connect Zn to the multimeter COM (black) and Cu to VΩmA (red).
- Record the open-circuit voltage.
- Briefly connect the LED + resistor in series.
Identify the anode (the electrode where oxidation takes place) and cathode (the electrode where reduction takes place), show the direction of electron flow.
Construct other voltaic cells using metal strips and metal salt solutions.
- Available substances
- Zinc foil, zinc sulfate
- Copper foil, copper(II) sulfate
- Lead foil, lead(II) nitrate
- Iron rods, iron(II) sulfate
- Magnesium ribbon, magnesium sulfate
Representing Electrochemical cells
We can use a circuit convention, i.e. an agreed way to represent the physical apparatus used to generate electricity from the electrochemical cell. By convention the half-cell in which oxidation takes place is written first, followed by the salt bridge and then the half-cell in which reduction takes place. For the zinc|copper electrochemical cell the oxidation takes place in the zinc|zinc sulfate half-cell.
Zn|Zn2+ ∥ Cu2+|Cu
Oxidation reaction || Reduction reaction
Explaining the chemistry
The anode is the electrode where oxidation takes place. In this case, electrons are removed from the more reactive metal.
Zn(s) → Zn2+(aq) + 2e
The cathode is the electrode where reduction takes place. In this case, electrons are given to the ions of the less reactive metal to produce the metal again.
Cu2+(aq) + 2e → Cu(s)
The cell reaction
Cu2+(aq) + Zn → Zn2+(aq) + Cu(s)
The cell convention
Cell notation: Zn(s) | Zn2+(aq) ∥ Cu2+(aq) | Cu(s)
Left is the anode (oxidation), right is the cathode (reduction).
Summary
An electrochemical voltaic/galvanic cell converts chemical energy to electrical energy by coupling oxidation at the anode to reduction at the cathode.
Full, total ionic, and net ionic equations clarify which species change and which are spectators.
The salt bridge completes the circuit by allowing ions to move; nitrate or sulfate ions usually act as spectators.
Check your understanding
- Identify the anode and cathode in Zn|Zn2+ ∥ Cu2+|Cu, and state the direction of electron flow in the external circuit.
- Write the full, total ionic, and net ionic equations for the overall cell reaction. Which ions are spectators?
- Predict what happens to the voltage if the CuSO4 solution is diluted while ZnSO4 stays the same. Explain qualitatively.
Now test yourself
Click on the button below to access the self-tests for MYP9 and MYP10.